Contraindications
VALCHLOR is contraindicated in patients with known severe hypersensitivity to mechlorethamine. Hypersensitivity reactions, including anaphylaxis, have occurred with topical formulations of mechlorethamine.
Warnings And Precautions
- Mucosal or eye injury: Exposure of the eyes causes pain, burns, inflammation, photophobia, and blurred vision. Blindness and severe irreversible anterior eye injury may occur. Exposure of mucous membranes to mechlorethamine such as the oral mucosa or nasal mucosa causes pain, redness, and ulceration, which may be severe. Should eye exposure or mucosal contact occur, immediately irrigate for at least 15 minutes with copious amounts of water, followed by immediate medical consultation
- Secondary exposure: Avoid direct skin contact with VALCHLOR in individuals other than the patients due to risk of dermatitis, mucosal injury, and secondary cancers
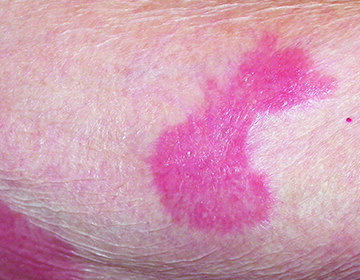
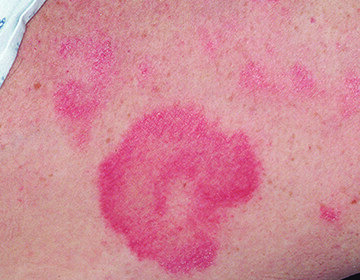
- Dermatitis: Dermatitis may be moderately severe or severe. Monitor patient for redness, swelling, inflammation, itchiness, blisters, ulceration, and secondary skin infections. Stop treatment with VALCHLOR or reduce dose frequency
- Non‑melanoma skin cancer: Monitor patient during and after treatment with VALCHLOR
- Embryo‑fetal toxicity: May cause fetal harm. Women should avoid becoming pregnant while using VALCHLOR due to the potential hazard to the fetus
- Flammable gel: VALCHLOR is an alcohol‑based gel. Avoid fire, flame, and smoking until the gel has dried
Adverse Reactions
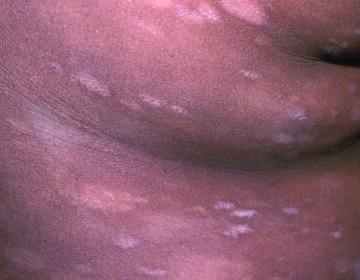
The most common adverse reactions (≥5%) were dermatitis (56%), pruritus (20%), bacterial skin infection (11%), skin ulceration or blistering (6%), and hyperpigmentation (5%). These reactions may be moderately severe or severe. Elderly patients aged 65 and older were observed to be more susceptible to experiencing cutaneous adverse reactions. Depending on severity, treatment reduction, suspension, or discontinuation may be required.
Use In Specific Populations
- Lactation: There are no data on whether mechlorethamine or its metabolites are present in breast milk or how they affect the child or milk production. Due to the risk of topical or systemic exposure from the mother’s skin and the potential for serious adverse reactions, advise patients not to breastfeed during treatment with VALCHLOR
- Contraception: Females who are able to become pregnant, and males with female partners who are able to become pregnant, should use a barrier method of contraception to avoid direct exposure of reproductive organs to VALCHLOR
- Infertility: The reproductive effects of VALCHLOR have not been studied; however, systemically administered mechlorethamine may impair fertility. The reversibility of the effect on fertility is unknown
Dosing and Application
VALCHLOR is for topical dermatologic use only. Apply a thin film of gel once daily to affected areas of the skin. VALCHLOR is a cytotoxic drug and special handling and disposal procedures should be followed during use. Caregivers must wear disposable nitrile gloves when applying VALCHLOR. Patients and caregivers must thoroughly wash hands after handling or applying VALCHLOR.
To report SUSPECTED ADVERSE REACTIONS, contact Helsinn Therapeutics (U.S.), Inc. at 1-855-482-5245 or FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Please see the VALCHLOR full Prescribing Information and Medication Guide (EN/ES).